About Te Rēhita Mate Ūtaetae

Once a patient is diagnosed with breast cancer, their information is included in Te Rēhita Mate Ūtaetae - Breast Cancer Foundation National Register.
This data is used for research, to audit care, to help plan breast cancer services, and to identify where further progress is needed to improve the lives of everyone affected by breast cancer, now and in the future.
Key facts
- Te Rēhita Mate Ūtaetae - Breast Cancer Foundation National Register holds a rich database of over 45,000 patients who have been diagnosed with invasive and pre-invasive breast cancer between 2000 and 2023. Approximately 4,000 new patient registrations are added each year.
- Data on Te Rēhita Mate Ūtaetae has been collected since 2000 for use in research and audit to help improve diagnosis, treatment and outcomes.
- From 1 January 2020, Te Rēhita Mate Ūtaetae includes all patients diagnosed with pre-invasive and invasive breast cancer in New Zealand from Northland to Southland.
- Of the patients in these regions, more than 99% are included in Te Rēhita Mate Ūtaetae (less than 1% opt out).
- New Zealand is a world leader in the collection of near-national data about advanced breast cancer (ABC) diagnosis and treatment. Source: BCFNZ Study into metastatic / advanced breast cancer.
What type of information is on Te Rēhita Mate Ūtaetae?
Te Rēhita Mate Ūtaetae collects information on
- Demographics (NHI, name, age, ethnicity, postcode)
- Diagnosis (breast symptoms and referral)
- Family history and risk factors
- Faster cancer treatment
- Surgery
- Histology
- Therapies
- Follow-up
- Loco-regional recurrence
- Advanced breast cancer
Te Rēhita Mate Ūtaetae demographics
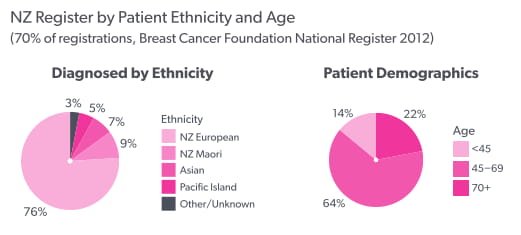
Based on Te Rēhita Mate Ūtaetae data analysed in 2012:
- The majority of breast cancer patients (64%) are aged between 45 and 69 years old.
- 76% of breast cancer patients are NZ European, 9% are NZ Māori, 7% are Asian and 5% are Pasifika.
Every two years, Te Rēhita Mate Ūtaetae report will highlight key statistics and trends and investigate select questions from NZ clinicians and the public.
To read published reports generated from Te Rēhita Mate Ūtaetae data click below.
How Te Rēhita Mate Ūtaetae data is used
Data from Te Rēhita Mate Ūtaetae is used for:
- Research
- Epidemiology and changing patterns of breast cancer
- Assessing efficacy and treatment of existing and new treatments
- Identifying regional and NZ-specific cancer trends
- Assessing and addressing inequities in care
- Indirectly enabling other research, e.g. clinical context for cancer genome research
- Healthcare planning
- Audits for quality measurement and improvement, e.g. BreastSurgANZ Quality Audit
Data collection
Data from breast cancer patients in both public and private healthcare systems is collected from hospitals and health professionals by authorised data managers.
The more complete the data, the greater the value of Te Rēhita Mate Ūtaetae for improving treatment and care in the future. Therefore, patients are followed up all their lives. If needed, general practitioners (GPs) may also be contacted by an authorised data manager for annual follow-up data, including breast cancer medications prescribed and any recurrent or new breast cancers.
Te Rēhita Mate Ūtaetae is an opt-out database. If you do not want to be included please click here.
Ethics approval
Te Rēhita Mate Ūtaetae - Breast Cancer Foundation National Register, operates in compliance with our ethics approval 16/NTA/139/AM03, The Privacy Act 2020, the Health Information Privacy Code and the principles of Te Tiriti o Waitangi / Māori data sovereignty.
Ethics approval is provided by the Health and Disability Ethics Committee (HDEC). The Health and Disability Ethics Committee (HDEC) is a Ministerial committee (established under section 11 of the New Zealand Public Health and Disability Act), whose function is to secure the benefits of health and disability research by checking that it meets or exceeds established ethical standards. For more information, visit the Health and Disability Ethics Committee website.
All data requests are reviewed for ethics approval before release. Only de-identified data is released. For more information see Data Request.
